What is Periodic Medical Device Testing?
Guaranteeing patient’s safety and keeping the effectiveness of medical equipment is crucial through regular medical device tests.healthcare institutions in the United States must regularly conduct examinations and assessments of their health equipment to make sure correct operation and adherence to essential standards.
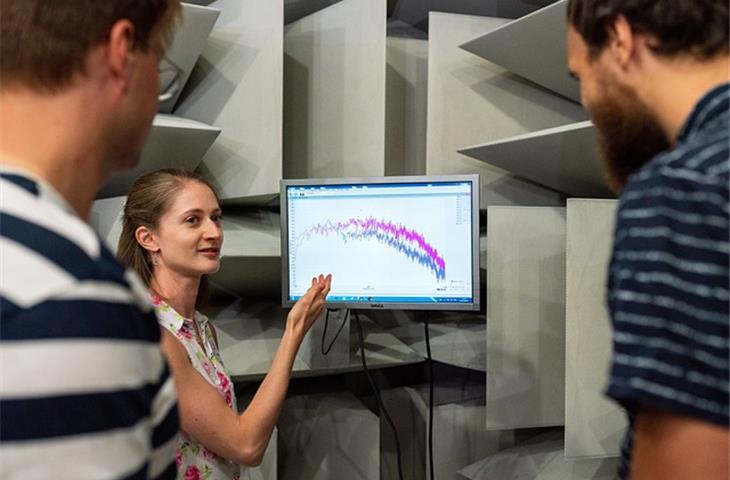
The article delves into the importance of regular medical device tests by highlighting four key requirements and providing detailed discussions of their significance.Guaranteeing compliance with regulatory standards is one of the primary reasons for regular medical device tests.In the U.S., patient’s safety is ensured by healthcare institutions adhering to guidelines and regulations under the oversight of the FDA (U.S. Food and Drug Administration) (FDA), which approves and monitors health equipment.

through routine testing, any variations from these standards can be identified, enabling prompt corrections and the avoidance of potential injury to patients.Early detection of equipment failures is vital for the avoidance of incidents and harm.Facilities can ensure ideal operating conditions for health equipment through frequent inspections, thereby reducing the probability of failures and Enhancing patient care outcomes.
Operation and calibration of medical devices is also a critical function performed by frequent inspection.Over time, the accumulation of silt, waste, or fraying and deterioration can impact operation of devices.The training and education of medical workers is another important aspect of periodic medical device testing.
medical workers, by understanding the importance of frequent inspection and the correct methods for conducting inspections, can contribute to the general safety and effectiveness of medical devices.In the following sections, we will provide insights into the significance of periodic medical device testing and its effect on patient treatment and security by exploring each of these requirements in detailed examination.
Maintaining client security and ensuring the excellence of treatment is essential for U.S. Medical care units, which are subject to stringent regulations regarding the deployment of medical gear and adherence with these standards.By frequent inspection, Medical care units can verify adherence of their devices with these standards, thus reducing the chance of patient injury and legislative consequences.
Early detection of issues can protect lives and stop expensive fixes or substitutes.Upkeep, however, includes cleaning, applying lubrication, and examining equipment to avert possible problems.The material of instructional modules must include multiple perspectives of the health care system equipment evaluation, which includes the following:
Investing in in education and training can improve the general quality of the routine healthcare equipment testing initiative for medical facilities.This initiative improves patient treatment and adds to the general quality of the health care system in the U.S..




